Difference between revisions of "Basic electricity:1.2 Electric Charges"
m (Admin moved page Basic electricity:Electric Charges to Basic electricity:1.2 Electric Charges) |
Revision as of 08:45, 29 December 2021
1.2 Electric Charges
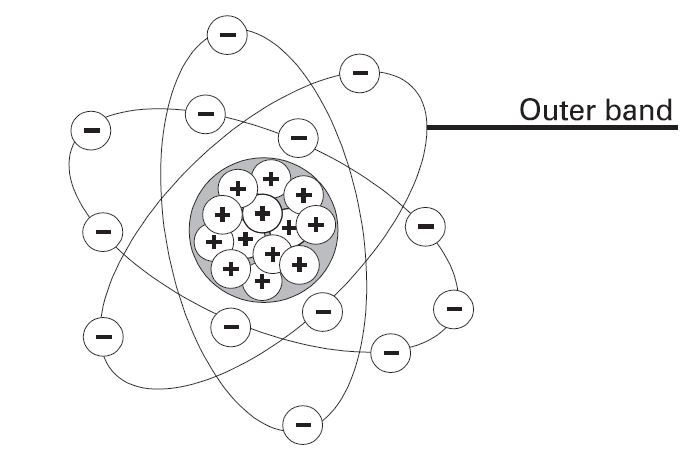
Neutral state of an atom
Elements are often identified by the number of electrons in orbit around the nucleus of the atoms making up the element and by the number of protons in the nucleus. A hydrogen atom, for example, has only one electron and one proton. An aluminum atom (illustrated) has 13 electrons and 13 protons. An atom with an equal number of electrons and protons is said to be electrically neutral.
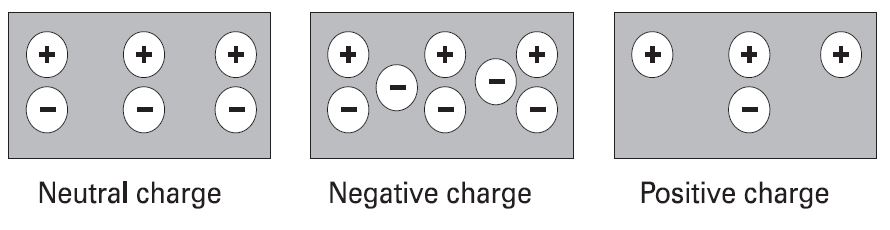
Positive and negative charges
Electrons in the outer band of an atom are easily displaced by the application of some external force. Electrons which are forced out of their orbits can result in a lack of electrons where they leave and an excess of electrons where they come to rest. The lack of electrons is called a positive charge because there are more protons than electrons. The excess of electrons has a negative charge. A positive or negative charge is caused by an absence or excess of electrons. The number of protons remains constant.
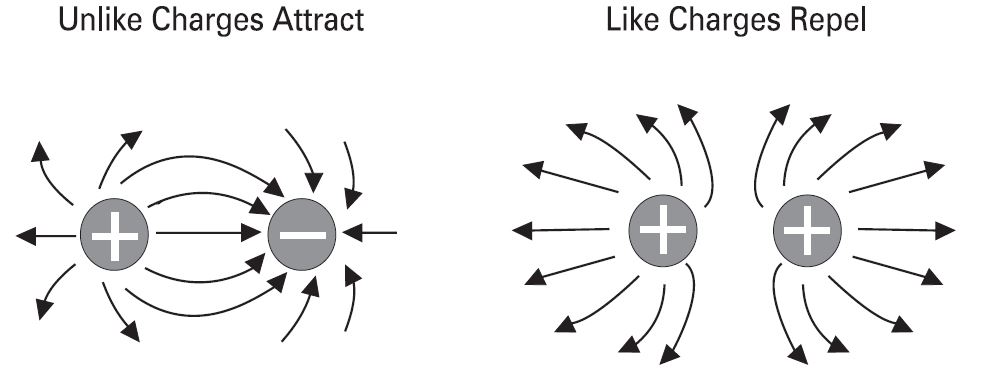
Attraction and repulsion of electric charges
The old saying, "opposites attract" is true when dealing with electric charges. Charged bodies have an invisible electric field around them. When two like-charged bodies are brought together, their electric field will work to repel them. When two unlike-charged bodies are brought together, their electric field will work to attract them. The electric field around a charged body is represented by invisible lines of force. The invisible lines of force represent an invisible electrical field that causes attraction and repulsion. Lines of force are shown leaving a body with a positive charge and entering a body with a negative charge.
Coulomb's Law
During the 18th century a French scientist, Charles A. Coulomb, studied fields of force that surround charged bodies. Coulomb discovered that charged bodies attract or repel each other with a force that is directly proportional to the product of the charges, and inversely proportional to the square of the distance between them. Today we call this Coulomb's Law of Charges. Simply put, the force of attraction or repulsion depends on the strength of the charged bodies, and the distance between them.