Difference between revisions of "Basic electricity:1.0 Introduction"
| Line 22: | Line 22: | ||
=== Elements of an atom === | === Elements of an atom === | ||
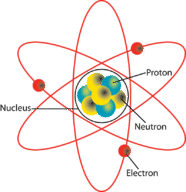
All matter is composed of molecules which are made up of a combination of atoms. Atoms have a nucleus with electrons orbiting around it. The nucleus is composed of protons and neutrons (not shown). Most atoms have an equal number of electrons and protons. Electrons have a negative charge (-). Protons have a positive charge (+). Neutrons are neutral. The negative charge of the electrons is balanced by the positive charge of the protons. Electrons are bound in their orbit by the attraction of the protons. These are referred to as bound electrons. | [[File:Atom.png|left|Elements of an Atom]] All matter is composed of molecules which are made up of a combination of atoms. Atoms have a nucleus with electrons orbiting around it. The nucleus is composed of protons and neutrons (not shown). Most atoms have an equal number of electrons and protons. Electrons have a negative charge (-). Protons have a positive charge (+). Neutrons are neutral. The negative charge of the electrons is balanced by the positive charge of the protons. Electrons are bound in their orbit by the attraction of the protons. These are referred to as bound electrons. | ||
=== Free electrons === | === Free electrons === | ||
Revision as of 09:14, 14 December 2021
Objectives
Welcome to the Basic Electricity series. It covers discussions about electricity and is designed to prepare you to better understand electricity.
- Explain the difference between conductors and insulators
- Use Ohm's Law to calculate current, voltage, and resistance
- Calculate equivalent resistance for series, parallel, or series-parallel circuits
- Calculate voltage drop across a resistor
- Calculate power given other basic values
- Identify factors that determine the strength and polarity of a current-carrying coil's magnetic field
- Determine peak, instantaneous, and effective values of an AC sine wave
- Identify factors that affect inductive reactance and capacitive reactance in an AC circuit
- Calculate total impedance of an AC circuit
- Explain the difference between real power and apparent power in an AC circuit
- Calculate primary and secondary voltages of single-phase and three-phase transformers
- Calculate kVA of a transformer
The objectives listed above may sound strange to you. You may also wonder why you would need to know these things to sell Siemens Energy & Automation products. Developing a basic knowledge of electrical concepts, however, will help you to better understand customer applications. In addition, you will be better able to describe products to customers and determine important differences between products.
Electron Theory
Elements of an atom
All matter is composed of molecules which are made up of a combination of atoms. Atoms have a nucleus with electrons orbiting around it. The nucleus is composed of protons and neutrons (not shown). Most atoms have an equal number of electrons and protons. Electrons have a negative charge (-). Protons have a positive charge (+). Neutrons are neutral. The negative charge of the electrons is balanced by the positive charge of the protons. Electrons are bound in their orbit by the attraction of the protons. These are referred to as bound electrons.
Free electrons
Electrons in the outer band can become free of their orbit by the application of some external force such as movement through a magnetic field, friction, or chemical action. These are referred to as free electrons. A free electron leaves a void which can be filled by an electron forced out of orbit from another atom. As free electrons move from one atom to the next an electron flow is produced. This is the basis of electricity.
Conductors, Insulators and Semiconductors
Conductors
An electric current is produced when free electrons move from one atom to the next. Materials that permit many elec- trons to move freely are called conductors. Copper, silver, aluminum, zinc, brass, and iron are considered good conductors. Copper is the most common material used for conduc- tors and is relatively inexpensive.
Insulators
Materials that allow few free electrons are called insulators. Materials such as plastic, rubber, glass, mica, and ceramic are good insulators.
An electric cable is one example of how conductors and insulators are used. Electrons flow along a copper conductor to provide energy to an electric device such as a radio, lamp, or a motor. An insulator around the outside of the copper conductor is provided to keep electrons in the conductor.